Question 62: What are causes of foaming in crude pre-flash drums and towers, and what options are available to mitigate foaming?
SHELTON (KBC Advanced Technologies, Inc.)
Surfactants cause foaming. Mike will discuss surfactants and amines that should not be in the crude. Sodium naphthenate is a common surfactant produced by the reaction of caustic injected at the desalter effluent and naphthenic hydrocarbons.
A simple solution is to move the injection downstream of the pre-flash or pre-fractionator to the bottom pumps. If the injection point is at the desalter effluent, solids and corrosion products can cause foaming.
Improving desalter solids removal will mitigate foaming. Precipitated asphaltenes that frequently occur with bitumens and asphaltic crudes can also cause foaming, so we would evaluate crude compatibility in that case.

The question includes pre-flash drums and towers, which I assume is a pre-fractionator. These applications are quite different in design and operation. In our pre-fractionator designs, we consider C factors, internals, tray design, and tower loadings.
We have used pre-flash drums in our latest grassroots designs because the hot trains have been so efficient that crude heater inlet temperatures are 600ºF to 610ºF. A flash drum removes water and requires lower pressure to suppress vaporization at the end of the hot train. The flash drum design avoids elevated pressures in the hot train and 900-pound flanges. Obviously, we specify vertical versus horizontal. We consider height versus diameter and liquid superficial velocity versus vapor velocity to optimize the ratio. We also consider disengaging height and the feed distributor inlet design. Of course, temperature and pressure have a major impact.

The pre-flash drum performs two functions: flashing water and suppressing vaporization. Many pre-flash drums are operated to remove light hydrocarbons. However, water causes vaporization, and operating pressure and temperature determine the vapor rate and composition. It is important to model the optimum pressure. Operate at the pressure required to remove water and not generate excessive hydrocarbon vapor load, which can result in carryover of bottoms.
In our designs, flashed vapors are sent to the flash zone. Designs where the flashed vapors are introduced in higher sections of the column can create problems. For any design, in the event of a foamover, temporarily increase pressure. With the flash drum, increase pressure until there is no vaporization. That will stop the foamover. It is important to have a pressure controller on that vapor line to the flash drum.

Pre-fractionator foaming is less likely because it is a refluxed column with an overhead product. The trays mitigate foaming, and the liquid loading should tend to knock down the foam. Again, in the event of a foamover, you could temporarily increase pressure. This may not be obvious, but we try to design for higher temperatures to reduce surface tension, which also mitigates foaming. In a new design, the pre-flash drum operating temperature is determined by the location in the hot train. Finally, improving desalter operation will mitigate foaming in the downstream columns.

BASHAM (Marathon Petroleum Corporation)
I want to reinforce some of Al’s points here. As he already mentioned, pre-flash tower or vessel foaming is a function of crude type salt or water carryover, temperature, and caustic addition. You are always going to have foaming occurring in a pre-flash drum or tower. The key here is to manage the foam and keep it in the tower. You must have sufficient vessel height and diameter necessary to disengage the foam. As Al also mentioned, the liquid superficial velocity is the key design parameter. It is important to keep in mind that the smaller the diameter of the vessel, the larger the foam height; so in narrow vessels, the liquid superficial velocity will need to be low in order to keep the foam height low. It is possible to add silicone-based antifoam to the pre-flash drum or tower, but consideration needs to be given to the downstream, gasoline, and distillate hydrotreater reactor catalyst.

DION (GE Water & Process Technologies)
Al and Kevin covered operational and mechanical issues regarding foam. Part of the question asked about the causes of foaming. There are surfactants in crude oil. Surfactants can be any organic molecule that has an atom that is not carbon or hydrogen, such as organic acids, organic amines, mercaptans, and other molecules with a polar group associated with them.

RUSSELL STRONG (Champion Technologies)
I have heard several comments that silicone antifoams in crude are problematic. There have been recent events offshore in the Gulf where so much antifoam was being used upstream that it was actually poisoning hydrotreater catalyst in the refinery from the upstream application. Other causes of silicone contamination can come from the crude while trying to control foaming in a flash drum or in a crude tower. To control those, silicone antifoams are sometimes used with occasional success. Several years ago, at a refinery down in the Houston, Texas area, I encountered severe foaming in a crude tower that would not go away. Standard silicone antifoams did nothing to solve the problem, but a fluorosilicone antifoam worked well. It was far more efficient and actually worked where the polysiloxane was deficient. It also offered less risk of downstream silicon contamination. So, keep this in mind as an option if you have crude unit foaming.
STEVEN FISCHER (Delek Refining)
At a previous refinery, we reintroduced the vapors to the flash zone with the result being quench to the flash drum that resulted in poor cutpoints. When we introduced the flash vapors from the flash drum to the flash zone, we saw that that the flash drum had actually acted like a quench, which could result in a poor cutpoint at the bottom of the crude tower.
SHELTON (KBC Advanced Technologies, Inc.)
Simulations do not indicate flash zone quenching if, as previously mentioned, the flash drum operating pressure is optimized to flash-only water. We have evaluated the flow schemes in models with the two streams mixed outside of the column and combined in the flash zone, but we get the same overflash.
STEVEN FISCHER (Delek Refining)
That was our assumption when we designed it that way, but our performance did not show that result. Our performance improved when we introduced it higher up.
ANDREW SLOLEY (CH2MHILL)
Addressing that last comment, I think what you are seeing there, when you see the poor performance, is the mixing of transfer line liquid with the vapor coming in, which is an issue with the equipment and not having the vapor segregated from the transfer line.
SHELTON (KBC Advanced Technologies, Inc.)
Our designs do have a separate flash drum vapor nozzle in the flash zone. It is important to have a pressure controller on the flashed vapor line, so the drum is not riding on the lower flash zone pressure. I do not know if that is your case or not. Do you have pressure control on the pre-flash drum? If not, a large pressure drop will produce a very high vapor rate, and then hydrocarbons will be flashed. In that case, there could be some quenching. We try to just flash the water and no hydrocarbons. When you think about it, if there were substantial light hydrocarbons, the desalter would overpressure. So, there are not a lot of light hydrocarbons in the crude because the only difference in the flash drum versus desalter operation is the desalter pressure, which is also low compared to the elevated hot train pressure.
STEVEN FISCHER (Delek Refining) We had some light hydrocarbons going overhead in addition to water.
SHELTON (KBC Advanced Technologies, Inc.)
There may also be recycle streams quenching the flash zone.
ROBERTSON (AFPM) Al, could you comment on the superficial velocity?
SHELTON (KBC Advanced Technologies, Inc.)
Liquid superficial velocity is a function of the vessel height versus diameter and design of the drum, which differs for vertical versus horizontal vessels. It is specific to each design and not a variable for an existing drum. Pressure is the important operating variable. If there is no pressure controller on the vapor from the flash drum, then that deficiency can be remedied online because there is usually a block valve at the column. In that case, the back pressure controller can be installed online.
VILAS LONAKADI (Foster Wheeler USA Corporation)
Is there any experience with the use of any internals in the pre-flash drums?
SHELTON (KBC Advanced Technologies, Inc.)
There are several types of feed distributors, including vortex tube clusters (VTC) and tangential nozzles. There are many effective feed distributors that will improve disengaging.
VILAS LONAKADI (Foster Wheeler USA Corporation)
Not about just the feed entry, but in the drum itself.
SHELTON (KBC Advanced Technologies, Inc.)
We do not recommend demisters on vapor outlets, and flash drums do not typically have any internals.
VILAS LONAKADI (Foster Wheeler USA Corporation)
Some vendors offer vortex tube clusters. I want to know if anyone has used them.
SHELTON (KBC Advanced Technologies, Inc.)
Yes, we mentioned vortex tube clusters (VTC), which have been used successfully in drums that operate at high velocities. We have also seen VTC distributors used for revamps to increase throughput at higher drum velocities. They have been very effective.
VILAS LONAKADI (Foster Wheeler USA Corporation)
Did it reduce foaming?
SHELTON (KBC Advanced Technologies, Inc.) Yes, VTC distributors have been used to solve foaming problems for existing vessels.
SHELTON (KBC Advanced Technologies, Inc.)
Foaming in flash drums and pre-fractionators is often caused by crude contaminants. Inorganic fines (sand, corrosion products, etc.), precipitated asphaltenes and sodium naphthenates formed from the reaction of caustic and naphthenic hydrocarbons have been identified as precursors. If caustic is injected at the desalter effluent, a simple solution is to move the caustic injection downstream of the flash drum to the pre-flash bottoms or hot train pumps.
The immediate solution to a foaming problem is to increase pressure to decrease vaporization. In a prefractionator, in addition to increasing pressure, higher reflux or wash rates will tend to knock down the foam front. Increasing temperature will reduce surface tension and mitigate foaming. Long term solutions include improving desalter operation (particularly solids removal) and improved selection of treating chemicals for the preheat train and desalters.
The design and operation of pre-flash drums and refluxed pre-fractionator columns are different. Vessel design (vertical versus horizontal) and disengaging height affect foaming. KBC design guidelines for pre-flash drums include height versus diameter, liquid superficial velocity versus vapor velocity, disengaging parameters, feed distributors and pressure. For any design, increasing operating pressure will reduce foaming.
Pre-flash drums are located in the hot crude train downstream of the desalters to flash water and suppress vaporization at the end of the hot train. Flash drum vapors on pressure control are routed to the crude column flash zone. Flash drum pressure sets the vapor rate and composition. Simulations show that water causes vaporization in heat exchanger services at the end of the hot train, not light hydrocarbons. Very light hydrocarbons would overpressure the desalters, if present. Simulations will determine the flash drum pressure required to remove dissolved water from the desalter effluent. The flash drum should be operated at the pressure required to remove water and no lower to reduce carryover of flashed crude. In the event of a foamover, the foam can be broken by temporarily increasing drum pressure to reduce vaporization. Good desalter operation with no water carryover to the flash drum will minimize foaming. Desalters should be operated with less than 0.5% BS&W in the effluent. Prefractionators are typically refluxed distillation columns with an overhead product such as light naphtha and may also have sidecuts. Foaming is less prevalent in a refluxed column. In the event of a foamover the foam front can be broken by first increasing reflux rate and if necessary, temporarily increasing overhead pressure.
BASHAM (Marathon Petroleum Corporation)
Foaming is always present in pre-flash drums and towers. It can be a function of several parameters including crude type, desalter performance (water carryover), drum or tower temperature, and caustic addition. Depending on its feed location in the atmospheric crude tower, pre-flash drum vapor can cause black distillate, black atmospheric gas oil, and increased atmospheric tower bottoms if the foam contains flashed crude. Similarly, in pre-flash towers foam with entrained flashed crude can cause black naphtha. The key to managing foam is keeping it in the pre-flash drum or tower.
A properly designed vessel (drum or tower) will allow sufficient height to disengage the vapor from the liquid. The most important design parameter is the superficial velocity of the flashed crude. The foam height is directly proportional to the liquid superficial velocity. The liquid superficial velocity must be sufficiently low enough to keep the foam height below the vapor outlet of the drum or tower. The foam height is also a function of the tower or drum diameter (cross-sectional area.): the smaller the diameter, the larger the foam height. This means that foaming will be a bigger concern in narrow vessels, so the liquid superficial velocity will need to be low in order to keep the foam height low.
It is possible to add silicone-based antifoam to the pre-flash drum or tower, but consideration must be given to downstream gasoline and distillate hydrotreater catalyst silicon loading.
LEE (BP Products North America)
A potential cause is water carryover out of the desalter that is vaporized in the flash drum. If there is water carryover and high shear stresses associated with a letdown valve with high pressure drop, this situation can generate small droplets which would contribute to foam generation. Foaming is often associated with high vapor rates, so a crude with a significant amount of vaporization at the flash drum conditions may have high potential for foaming. Antifoam use, and additional enhanced separations hardware, such as vortex cluster internals, can be considered.
DION (GE Water & Process Technologies)
Any organic molecules with atoms other than hydrogen or carbon are potential surfactants. Examples of such molecules are; alkyl phenols, organic amines, organic acids, and mercaptans. Foaming can be mitigated through the use of a defoamer or antifoam chemistry.Defoamers function by reducing the interfacial surface tension and viscosity. Antifoams function by modifying the interfacial surface elasticity. Most products commercially available from specialty chemical suppliers, such as GE Water & Process Technologies, function in both manners due to the behavior of their surfactant structure. The most effective defoamers in hydrocarbon environments are typically silicone based. If silicone poisoning is a concern, non-silicone-based defoamers, such as glycolic materials, are available.
BRUCE WRIGHT (Baker Hughes) Pre-flash tower foaming is most often caused by high solids loading coupled with high gas flows. Foam control with Baker Hughes Si-based antifoams has proven to be effective.
DENNIS HAYNES (Nalco Energy Services)
Crude viscosity, hydrocarbon polarity, solids content, caustic use, and vapor disengaging in flash sections and tower bottoms are discussed as causes for foaming. Antifoams have been around for quite a while that may be utilized in this area; however, the first step in corrective action is to determine that it is actually a stabilized foam layer and not tower flooding. There are instances where pre-flash towers are operated above design or have had some internal damage that causes flooding which is mistaken for foaming.
ANDREW SLOLEY (CH2M HILL)
One major cause of foam formation in these units is surface-active agents stabilizing the foam film on the liquid-vapor interface. Some of these agents are inherent components of specific crudes. However, many of them have been added to crude as well stimulation, drag-reducing, anticorrosion, or hydrogen sulfide scavenging additives. With continued production of heavier crudes and more aggressive well stimulation operations, foaming problems should be expected to get worse.
Solutions to foam formation include; antifoaming additives; foam-breaking inertial separators; and modifying operating conditions.
Silicone-based antifoaming additives can be effectively used. Their downside is that they vaporize and end up in the lighter products, particularly naphtha. This puts the antifoam into the downstream naphtha hydrotreater feed. Few hydrotreaters can tolerate this. Antifoams are rarely used.
Foam-breaking inertial separators have been used in a number of plants. They are derived from equipment design for oil production operations. In the oil fields they are proven technology. Experience in refineries, while limited, has been mostly successful. For certain plants and feeds they may be a choice worth serious consideration.
The most common method of avoiding foam-created problems has been to modify the plant operating conditions. This may include changes in feed rate, pressure, or temperature. Feed rate reduction increases effective residence time in equipment. It also reduces total vapor rate formation. While expensive, some plants are constrained to do this. Increasing pressure reduces vapor formation and increases vapor density. Both reduce the volume of vapor. Increasing operating pressure reduces foam problems. Temperature changes are more complex. Higher temperatures (at the same pressure) create more vapor volume, they also decrease liquid viscosity. These are competing changes. More vapor volume increases foam make. Lower viscosity speeds foam decay. In a plant with a foam problem, small temperature changes, in either direction, may help solve the problem. Experience has shown an operating temperature change as little as 10°F may change the vapor volume, or the viscosity, enough allow the flash drum or tower work, or be catastrophically worse.
Proper pre-flash installation includes balancing many factors including equipment size, expected operating conditions, and how to connect the pre-flash system to the existing unit. Revamps to add, or improve, pre-flash drums or towers need to be carefully evaluated.
Question 99: Tight oil-derived FCC feeds are known to contain high levels of contaminant iron (Fe) and calcium (Ca). What catalyst design features are important for minimizing their effects? What level of these contaminants can be tolerated? What lab procedures can accurately simulate Fe and Ca contamination?
KOEBEL (Grace Catalysts Technologies)
There are a lot of parts to this question, so I will respond to them independently. One of the catalyst design features that is important in any kind of feed, when you are going to get high iron and high calcium, is in the porosity. We talked a little before about how these contaminant metals tend to form these eutectics which can melt the surface of the catalyst and close off the pores. So having the right pore size distribution and mesoporosity in your catalyst is tremendously important. It is also really critical to look at the pore size distribution, not just the total pore volume; because in certain instances, you can have pore volume that measures consistently from catalyst to catalyst despite the difference in the pore size distribution. So I am sure all of the catalyst vendors will agree that the pore size distribution is a tremendously important piece of this puzzle.
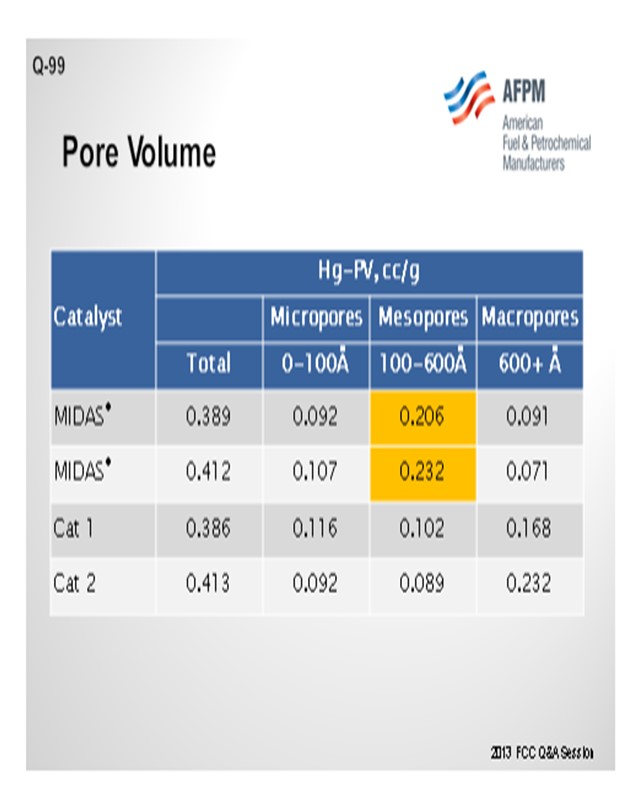
This slide shows an example of a commercial unit into which Grace put a MIDAS® catalyst. As I mentioned before, MIDAS® is a catalyst with which we take great care to optimize the pore volume and mesoporosity of the catalyst system. This particular unit ran relatively high iron plus calcium; and by putting higher mesoporosity into the system, we were able to help with the bottoms yield.
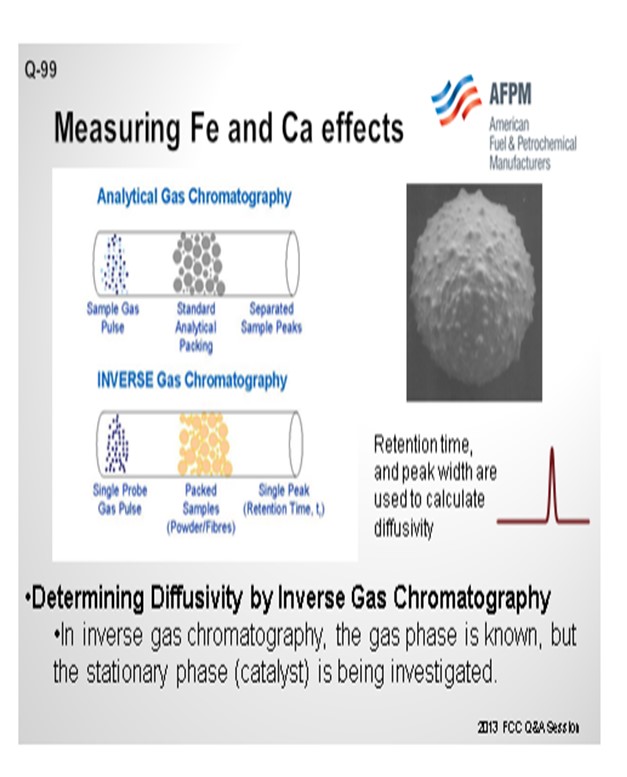
The question also asked about lab procedures we have found that simulate iron and calcium tolerance. Actual impregnation of iron and calcium on a fresh catalyst for lab deactivation is relatively difficult to accomplish. It is an ongoing area of research at Grace, but it is not one where we have found that the surface changes that happen to the FCC catalyst are easy to simulate in the lab. However, we do have a good test for actually measuring e-cat diffusivity. That test involves inverse gas chromatography where you take a gas chromatograph tube, pack it with FCC catalysts, and run a spike of a probe gas into it. If the FCC catalyst has a lot of diffusivity inherent in it, the probe gas will diffuse into and out of the catalyst sample that is in the chromatograph tube. It takes longer for the probe gas to come through in a more drawn-out profile on the other end, so you can measure the actual porosity of the catalyst sample quite well.
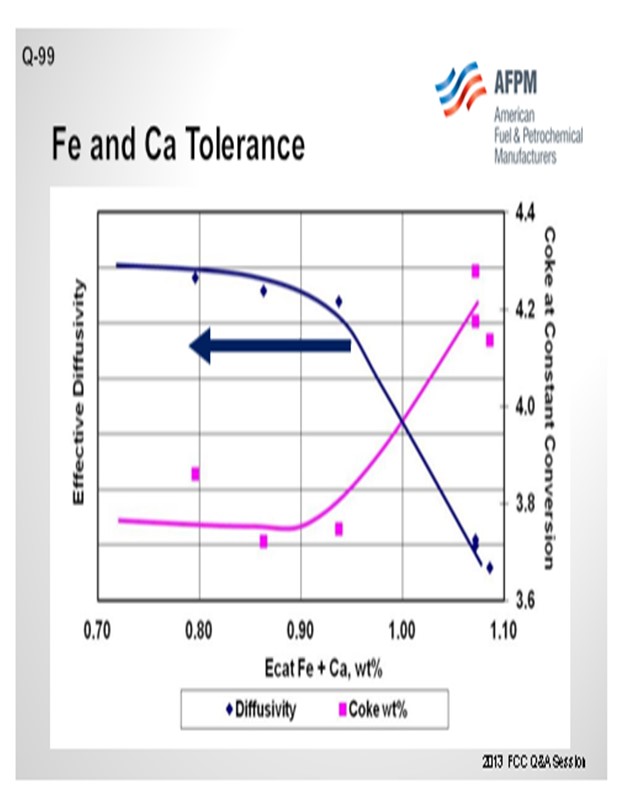
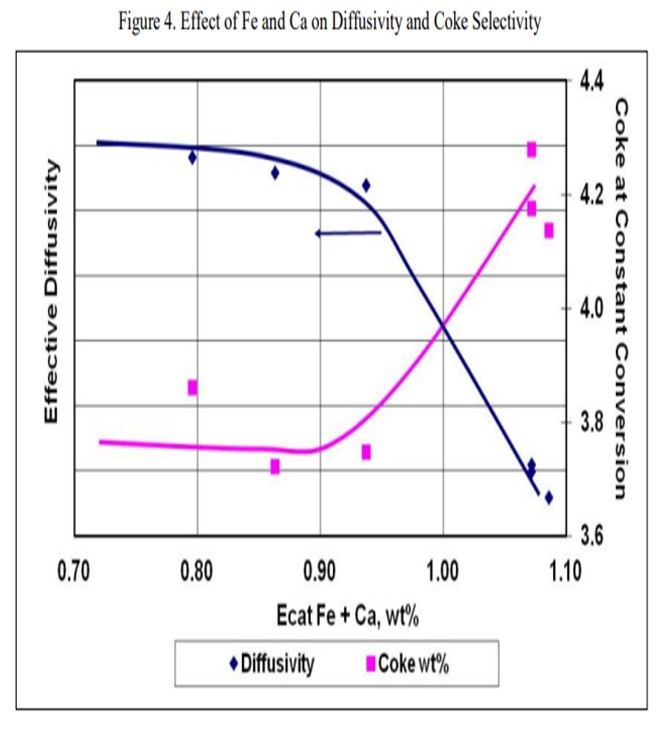
The next slide displays an example of the data from that type of testing and is representative of what we see across most samples. The blue line represents the effective diffusivity of the catalyst. As you pile on iron plus calcium, the diffusivity does not really change much until you fall off a cliff. In this case, that cliff happens at about 0.95 wt% iron. At that point, the bottoms yield, and the coke yield increase significantly. The detrimental effects of the contamination will be readily apparent in the unit at this point, and you will be able to observe the formation of the iron nodules on the catalyst particles as well. The actual level at where this rapid increase happens is going to vary greatly depending on the FCC catalyst.
It is very important to look at the amount of contaminant iron and contaminant calcium that you have on your FCC catalyst because all FCC catalysts, as well as the raw materials, have some base level of iron and calcium. To say that a catalyst will see this happen at 0.7 or 0.8 wt% iron is not really representative. You need to look at the delta amount of contaminant iron and contaminant calcium you are piling onto the catalyst. Grace certainly has experience with FCC catalysts running 0.6 to 0.7 wt% of contaminant iron plus calcium, which would equate to about 1.1 to 1.2 wt% of total iron plus calcium on the e-cat, while still maintaining reasonable bottoms conversion with a catalyst with properly designed mesopores and mesoporosity.
KEN BRUNO (Albemarle Corporation)
We agree with the comment Jeff made that pore size distribution is important, but we take it one step further. We believe that it is not only the internal pore size distribution, but it is also about the diffusivity of your surface. Again, when we wrap all that together and look at the accessibility, particularly with iron and calcium, we get a lot of deposits on the surface. With these deposits, you plug the surface, create an outer shell, and really decrease the diffusion character of the surface. We can capture that by measuring the accessibility. The key to overcoming that is, again, a good catalyst with high accessibility.
BART de GRAAF (Johnson Matthey INTERCAT, Inc.)
This question, like the previous one, shows that shale oil offers a lot of challenges, one of which can be octane. For the past 10 years, various suppliers have offered octane-selective additives that do not increase LPG. Butylenes can be an issue; and butylene-selective additives have been available for many years. Like Ken and Jeff said, various suppliers now offer catalysts with this selectivity in their base catalyst.
At Johnson Matthey, we spent a lot of time studying the effects of iron and calcium on the base catalyst. How does iron poisoning occur? We have one result we would like to share with you. One of the standard methods used to counter iron poisoning is adding e-cat next to the base catalyst. You are adding a lot of extra material to capture the iron and the calcium. You hope that in the end, by adding sufficient extra material, you will get just under the limit (of iron) that will seal off all of your catalyst pores. When you examine the catalyst or e-cats of a unit that has an iron poisoning problem and add e-cat that already contains a high iron content, you can see that the iron present on the base catalyst is slightly higher than you expected. Also, the iron present on the added e-cat is lower than anticipated from iron from the feed. This result suggests that although it was previously assumed that when you have an iron poisoned catalyst, nothing can be done to cure it, a minor amount of iron is mobile and can transfer by particle-to-particle interaction.
ROBERT “BOB” LUDOLPH [Shell Global Solutions (US) Inc.]
Question 29 of the 2006 NPRA Cat Cracking Seminar covered calcium effects on catalyst and equipment quite well. Catalyst porosity can have a profound effect on calcium and iron tolerance, “soaking up” nearly twice the base level if you significantly increase your porosity. But the pace at which the iron or calcium builds on catalyst in your unit is also a player. If you maintain good control of the feed blend, then higher levels can be achieved; if feed calcium and iron swings widely, then your unit tolerance will be much lower.
JOE McLEAN (BASF Corporation)
There has been a lot of talk about iron, aside from tight oil, for many years. The connection between iron and tight oil is just a new wrinkle on an old theme. An additional effect is that iron does act as a CO promoter. In a full-burn unit, you probably do not care. But if you are in a partial-burn unit, the CO promotion effect can really play havoc with the heat balance; so that is another effect of iron that you have to take into account.
We talked about iron and calcium, but iron poisoning is much worse when calcium is present than when it is not. That just goes back to the composition needed to form these eutectics. On the surface, it is silica. To make a glass, you need the silica source; you need the alkali source; you need the metal. The three of them together can then play a role. Of course, all FCC catalysts have silica if they have zeolite in them. That is where it originates. So they are worse together than either one is by itself. If you are in partial-burn and trying to control the CO level, you will have more difficulty in a high iron poisoning system.
PAUL DIDDAMS (Johnson Matthey INTERCAT, Inc.)
An additional effect of iron is that at high enough levels, it may behave as a reverse SOx additive. Fresh iron coming into the unit with the feed is able to pick up H2S (hydrogen sulfide) in the riser and transport it to the regenerator where it is converted to SOx.
UNIDENTIFIED SPEAKER
To expand on what Bob said, one final effect of iron is that iron action, especially when just fresh, is at the hydrogenation catalyst. So you may see coke and other things happening with an increase in FCC dry gas. If you have a unit that is in some way limited on coke-bearing capacity, you may get some negative effects that are interpreted as catalyst poisoning; but in reality, it is related more to making additional coke from the fresh iron that you are bringing into the unit.
ROBERTSON (AFPM)
Before we get to Question 100, I want to recognize and thank Yvette Brooks. She works for AFPM. She sits up here for all four of the Q&A sessions and keeps the program flowing in order so that we can get the transcripts out quicker. Over the last three years, these transcripts have come out months earlier than in the past as a result of the work Yvette does up here to stay organized. So Yvette, thank you very much. I also want to recognize Wendy Hefter with DWH Office Services. She is not here today; however, she takes the materials that Yvette prepares onsite and then painstakingly produces the final transcripts that are distributed in March.
GIM (Technip Stone & Webster)
My understanding is, with iron poisoning, that there is a distinction between organic and inorganic iron. It is the organic portion that causes the iron poisoning. Does anyone actually measure those two compounds differently or just total iron?
GEORGE YALURIS (Albemarle Corporation)
I am not familiar with a technique for measuring how much of the feed iron is molecular (organometallic), colloidal, or particulate. Usually, you can tell by examining the particles of the equilibrium catalyst after the iron has deposited on them. Different types of iron create different morphological features on the particle, so you can make conclusions from appearance differences when imaging the unit e-cat using the SEM/EDX technique. There is no clear size separation between the various types of iron. There is a continuum of iron that forms from molecular (organometallic)-type iron to colloidal and finely dispersed, all the way to particles which are up to catalyze size, are rich in iron, and come with the feed. The amount of destruction of the FCC catalyst iron will cause will depend on the size of iron species in the feed. The smaller, closer to molecular size the iron species are, the more destructive they will be the larger, the less likely they will cause any problems. I have seen units that have had very high iron; but because the iron was from large particulates, it had no effect on the catalyst performance.
JEFF KOEBEL (Grace Catalysts Technologies)
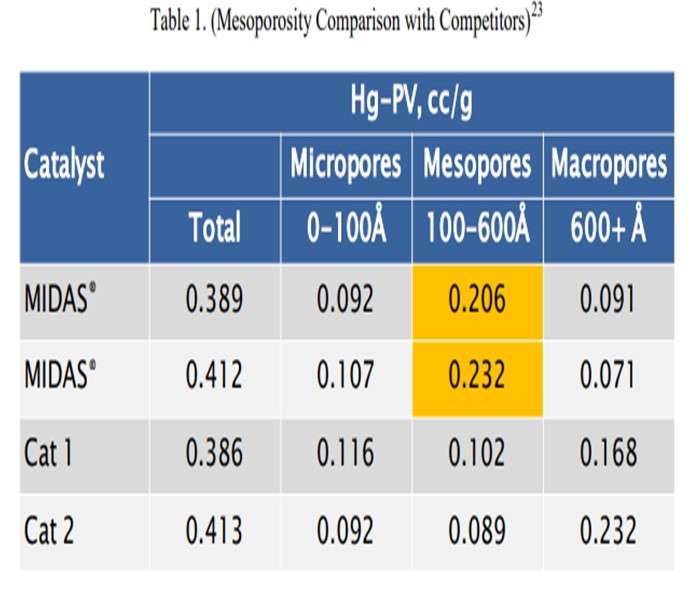
Catalyst design can be optimized to resist the effects of contaminant iron and calcium. High alumina catalysts, especially catalysts with alumina-based binders and matrices such as GRACE’s MIDAS® technology, are best suited to process iron- and calcium-containing feeds because they are more resistant to the formation of low-melting-point phases that destroy the surface pore structure.20 Optimum distribution of mesoporosity also plays a role in maintaining performance because diffusion to active sites remains unhindered, despite high-contaminant metals. Consider that while two catalysts may have similar total pore volume, their mesoporosity can vary greatly.21 MIDAS® catalyst was designed to maximize the abundance of mesopores or pores in the 100 Å to 600 Å size range. Table 1 shows the mercury pore size distribution of MIDAS® catalyst compared to competitive bottoms cracking catalysts.22 As can be seen here, even with similar total pore volume, MIDAS® technology has nearly twice the amount of pore volume in the 100 Å to 600 Å mesopore range compared to the competitive samples. This abundance of mesoporosity enables MIDAS® catalysts to more readily resist the poisoning effect of contaminant Fe and Ca.
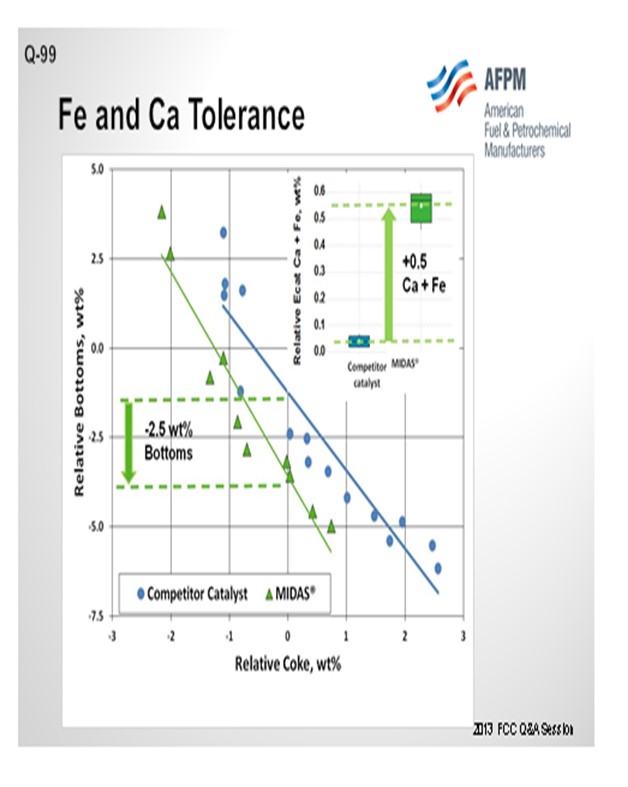
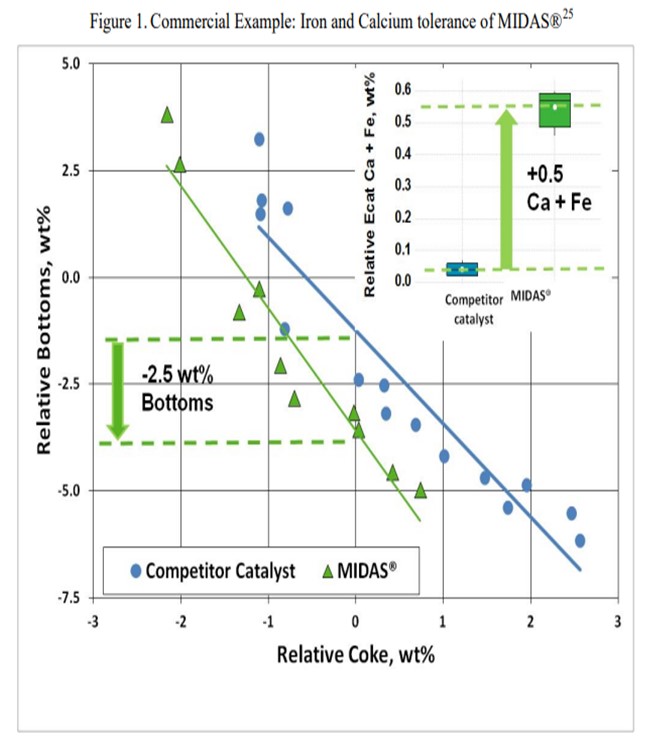
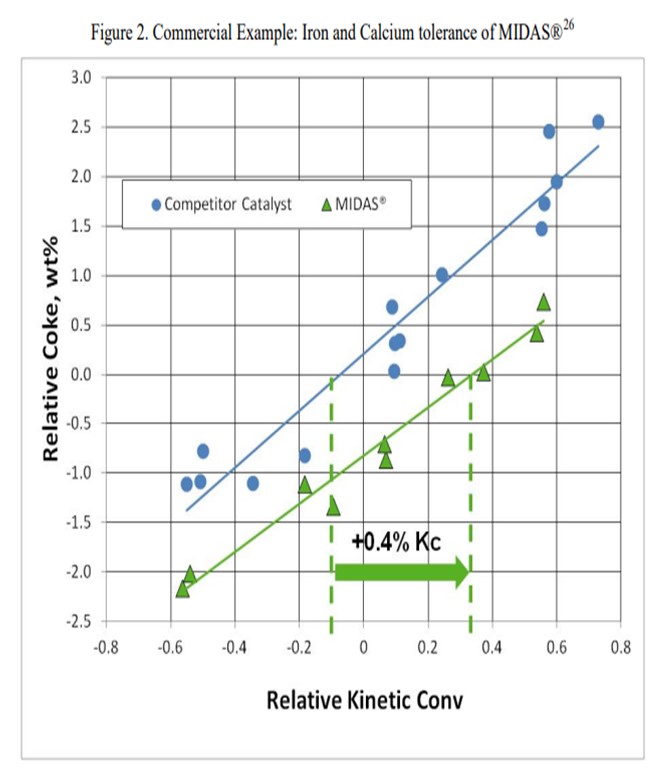
The resistance of MIDAS® to iron and calcium poisoning has been demonstrated in many commercial applications. Figure 1 and Figure 2 present the data from one such example. A refinery was processing a feedstock high in iron and calcium. Over time, the unit exhibited the symptoms of iron poisoning. Iron nodules built up on the catalyst surface and conversion, and bottoms cracking began to suffer. The catalyst was switched from a competitive catalyst to MIDAS®. Upon switching, activity, bottoms cracking, and coke selectivity improved despite the higher metal's levels.
To address the part of the question regarding lab simulation of Fe poisoning, it is not currently possible to simulate the full effect of Fe and Ca poisoning on FCC catalyst performance in laboratory scale deactivation. Iron poisoning causes changes in catalyst morphology and texture in the commercial unit with the formation of nodules on the surface. These nodules have been difficult to replicate in the lab. Laboratory simulation of iron deactivation is an area of ongoing research at Grace.
Impregnation with calcium can cause ion exchange in the zeolite, resulting in undesired changes in the catalyst unit cell size. Grace is working with spray coating techniques to better simulate calcium deposition.
Grace does have an available laboratory test method that can measure the effects of Fe and Ca poisoning. The diffusivity test can confirm adequate diffusion into and out of the pores of a sample of FCC e-cat. The diffusivity test is a proprietary method using inverse gas chromatography, which is depicted in Figure 3. A section of chromatography tube is packed with the e-cat sample. A pulse of a probe gas is shot into the tube, and the rate that the probe molecules pass through is related to the diffusivity. The probe gas molecules will go into the pores of the e-cat, so the rate they pass through will be slower and the diffusivity number will be high. For e-cat with plugged pores due to Fe or Ca contamination, the probe molecules will flow right through, and the diffusivity number will be lower.
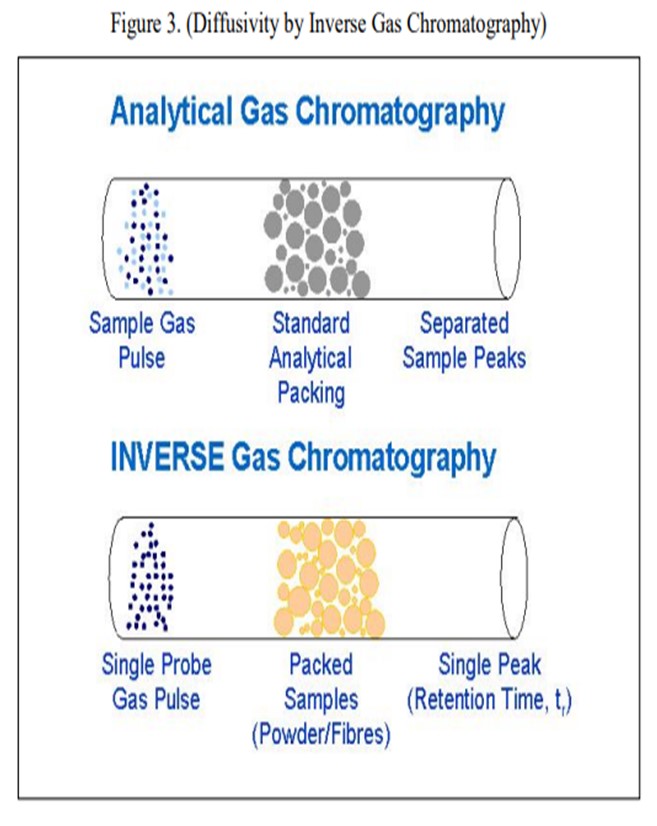
Figure 4 is an example illustrating the impact of equilibrium iron plus calcium on diffusivity. A drop in diffusivity results in an increase in coke at constant conversion. As these contaminants build up on the surface as rings, coke selectivity is lost due to mass transfer limitations, leading to higher rate of secondary reactions, e.g., coke.
'
The ability of a FCC catalyst formulation to tolerate Fe and Ca poisoning will vary greatly depending on the catalyst chemical makeup and the inherent porosity of the fresh catalyst. Grace has experience with units operating successfully with e-cat Fe in the range of 0.5 to over 1 wt%. A good general rule of thumb is that performance can begin to suffer with as little as 0.2 wt% incremental Fe if the catalyst is particularly prone to Fe poisoning.
PAUL FEARNSIDE (Nalco Champion Energy Services)
Increased iron (Fe) and calcium (Ca) removal can sometimes be accomplished across the desalters by carefully acidizing the desalter washwater. The acidizing essentially increases the solubility of both the Fe and Ca into the washwater for the increased removal.
CHRIS CLAESEN (Nalco Champion Energy Services)
If the largest part of the Fe is contained in the solids, the solids removal can be improved with a specific Nalco Champion solids wetting additive. Fe removal of over 90%, with desalted crude Fe levels below 0.5 ppm, has been achieved with this additive.
RAUL ARRIAGA and KEN BRUNO (Albemarle Corporation)
The critical catalyst feature for success with Fe and Ca poisoning is high accessibility, or in other words, a catalyst with superior internal and surface diffusional character. AMBER™ and UPGRADER™ were developed with high accessibility and are proven for tight oils. For additional insight, please see Albemarle’s answer to Question 98.
Regarding contaminant levels, with today’s technologies, an FCC catalyst can operate successfully with iron contents as high as 25,000 ppm and CaO (calcium oxide) as high as 28,000 ppm. Regarding laboratory procedures, Albemarle has developed a deactivation protocol to simulate the impact of high amounts of iron and calcium on a catalyst. The method is called CD-ALFA (Cyclic Deactivation with Accessibility Loss by Fe and Ca Addition) and was developed to simulate the effect of metal contaminants on accessibility. If a catalyst is not evaluated at its actual equilibrated accessibility, then the yields from performance testing are usually misleading. In nearly all cases, traditional deactivation methods, including cyclic deactivation and Mitchell/CPS, result in an Albemarle Accessibility Index (AAI) not reflective of its true value.