Question 45: Has bromide been reported in your hydroprocessing unit feeds or discovered as ammonium bromide in the reactor effluent exchangers? If bromide is present, do you use the same wash water practices for ammonium chloride deposition sufficient to avoid exchanger fouling? What are the possible sources of bromides
James Esteban (Criterion Catalysts & Technologies)
Bromine is in group 17 of the periodic table commonly known as Halogens. Halogen means “salt former”. It is this salt forming nature of the halogen group that makes them key in natural compounds. Halogen anions of Fluoride, Chloride, Bromide, and Iodide are present in living organisms. Further Bromide is present in all organisms, and organobromine is the most common organohalide. Marine organisms are the main source of organobromine compounds with the most abundant being methyl bromide (CH3Br) with an estimated 56,000 tons produced by marine algae each year.[1] The essential oil of the Hawaiian alga Asparagopsis taxiformis consists of 80% tribromomethane (bromoform).[2]. Since organobromine is common to all living organisms the presence of bromine in crude oil fractions is expected, but a study of relative concentrations in specific crude sources is not known.
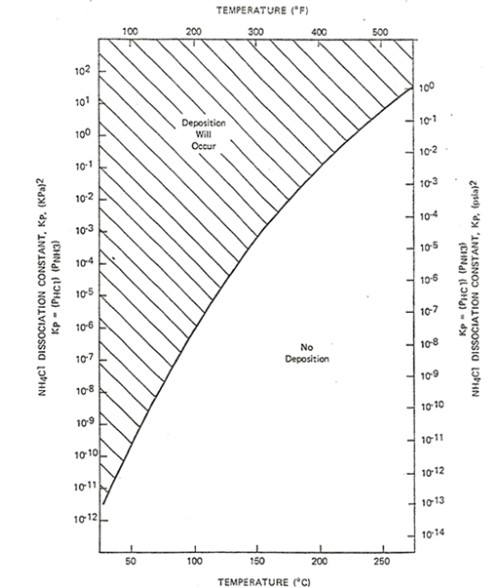
As such Bromine has indeed been reported in feeds to hydroprocessing units. Similar to feed compounds containing Chlorides, Bromide forms HBr when hydrotreated and this strong acid in the presence of NH3 will deposit as NH4Br salts. The deposition of both ammonium chloride and ammonium bromide salts in hydroprocessing units is a function of the partial pressure of NH3 and HCl or HBr as well as operating temperature. These salts typically are found in the effluent heat exchange train where successive cooling and separation stages for reactor effluent take place. The sequential reduction in temperature and gross separation of gases from liquids increase the partial pressure of these components while approaching the deposition temperature. The following chart provides a curve for the deposition of ammonium chloride salts as a function of partial pressure of NH3 and HCl. [3] A deposition chart for ammonium bromide salts is not available however monitoring for the symptoms of ammonium chloride and ammonium bromide salt deposition is the same. Monitoring the differential pressure of reactor effluent exchangers, hot separator overhead exchangers, and Reactor Effluent Air Coolers is a common approach for tracking and identifying the potential formation of these salts.
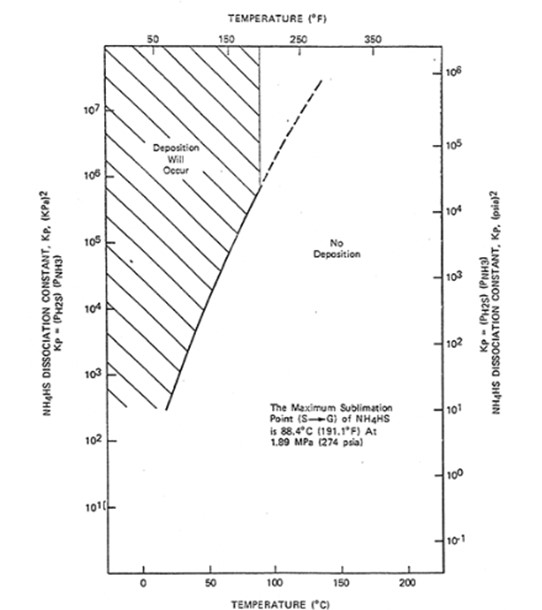
Salts formed in the effluent exchangers, hot separator overhead exchangers as well as Reactor Effluent Air Coolers are of great concern due to corrosion potential as well as build-up of pressure drop due to restricted flow paths limiting unit operating capability. In order to remove these salts water wash is required as specified by API 932. However, while deposition of ammonium chloride and ammonium bromide salts is similar to that of the better-known ammonium bisulfide it is not limited by a Maximum Sublimation Point under typical hydrotreater operations (refer to ammonium bisulfide deposition chart).[4] This leads to salt formations in locations outside of the traditional water wash locations. The use of intermittent water wash is recommended for the removal of these salts to prevent the build-up of excessive pressure drop.
It is critical when using intermittent water wash to remove the salt deposits completely as introducing water in the presence of these salts can result in excessive corrosion leading to potential loss of containment. It is recommended that experts in metallurgy, inspection, and mechanical integrity be consulted when considering solutions to address the potential formation of ammonium chloride and ammonium bromide salts. In addition, considerations for opening equipment must be addressed as the potential for cracking of certain materials is of concern.
Sam Lordo (NALCO Champion)
Bromine is mostly coming from production chemicals residuals in crude oil. HBr is a common well stimulation chemical and is typically removed in the production process, however, if removal is not complete it can be sent to the crude unit in the refinery. It will behave similarly as ammonium chloride; hence, water wash is an appropriate mitigation step. Chemical additive salt dispersants would also be effective.
Greg Savage (NALCO Champion)
Bromide salts were reported in the Rocky Mountain PADD IV region associated with processing locally produced crudes. Compounds containing Bromine are used in well drilling fluids and bromide is commonly found in salt water. Bromine is less reactive than chlorine but, like all halides, has the potential to form salts with cations.