Arriving at the scene, Dan takes in the chaotic view in front of him. A family’s home is ablaze, with smoke billowing out of the roof. He and his fire company have been dispatched to the two-story structural fire, and they run full-speed into the building – aware of the dangers ahead of them, but ready to risk their lives in the line of duty.
As Dan fights his way up the stairs, the floorboards – which are already severely burned out – collapse under his weight. He falls up to his armpits and becomes trapped in the mangled staircase, with fire blazing up from under him on the first floor of the home. Fearing the worst, Dan is certain that he will not come out unscathed.
“I was certain my legs were going to be burned,” he recalled in a recent interview about the incident.
Fortunately, Dan’s legs – and his life – were saved, thanks to the fellow firefighters that pulled him out of danger and his personal protective equipment (PPE), also known as turnout gear, gear that’s made possible by petrochemicals. In fact, over 80 percent of the materials used in PPEs originate from petrochemicals, from the mask that covers his face to the boots that protect his feet – and of course, the pants that cover his legs.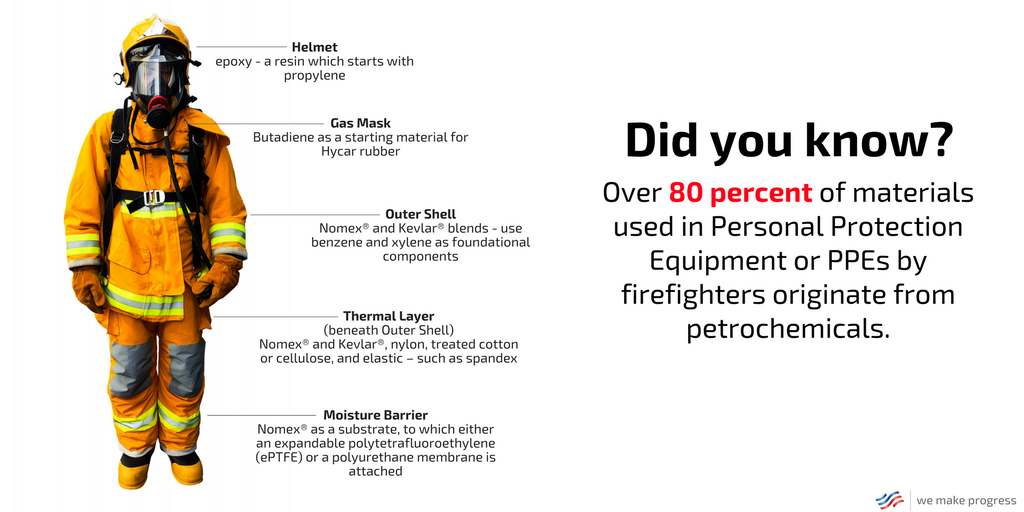
The outer shells of jackets and pants, for instance, typically consist of a Nomex® and Kevlar® blend – two aramid fibers that use benzene and xylene as foundational starting materials. Newer jackets increasingly incorporate polybenzoxazole (PBO) – an even stronger fiber, which starts with benzene and xylene as petrochemical building blocks.
Beneath the outer shell is a thermal layer, which is composed of many different yarns woven together. The most common are aramid fibers, such as Nomex® and Kevlar®, nylon, treated cotton or cellulose, and elastic – such as spandex. Nomex® is commonly used as the batting, surrounded by the multi-fiber fabric – and like the outer shell fiber, it requires benzene and xylene in the manufacturing process. Spandex, on the other hand, helps make PPE more comfortable and flexible. Spandex is created through a series of reactions that starts with either benzene or toluene, which is then reacted with a diol made from ethylene or propylene.
The moisture barrier consists of membranes of stretchable fabric, with pores small enough allow sweat to escape but prevent water from entering the suit. The moisture barrier relies on Nomex® as a substrate, to which either an expandable polytetrafluoroethylene (ePTFE) or a polyurethane membrane is attached. ePTFE is made starting with methane, which begins a series of chemical reactions to produce the end product. Polyurethane is made using benzene or toluene to create TDI or MDI, which is reacted with a diol – either ethylene glycol or propylene glycol.
Firefighters’ helmets typically consist of modern shells made from fiber reinforced epoxy – a resin that starts with propylene. The gas masks that protect their faces and respiratory systems rely on butadiene as a starting material for Hycar® rubber.
Finally, the boots that protect their feet from the elements consist of nitrile rubber outer soles with polyurethane-injected midsoles. CrossTech® liners are an ePTFE membrane in a Nomex® fabric. Even the laces are Nomex®.
As manufacturing techniques have advanced, PPE itself has improved over the years – so firefighters are facing substantially fewer injuries on the job.
According to the National Fire Protection Association, firefighter injuries decreased to 62,085 in 2015, from 103,340 in 1981. Burns accounted for only 7.5 percent of firefighter injuries in 2015; a statistic that would not be possible without the advanced and durable PPE that exists today.
For firefighters like Dan, PPE is so much more than a uniform to wear to work each day – it is gear that can save their lives. Without the petrochemical industry, the creation of this gear simply could not be possible. We are proud to play a key role in protecting our country’s firefighters and look forward to continuing to make key supplies for PPE companies advancing protective gear and technology.