Question 9: What operating conditions in a catalytic polymerization unit are conducive to the formation of esters? What is the main effect that esters have on unit performance?
KINDERMAN (Clariant Corporation)
The mechanism by which oligomerization takes place over the SPA catalyst necessitates that a phosphate ester is formed between the catalyst and the olefin during the reaction. The temperature required to disassociate the phosphate ester and convert the olefin depends on the olefin in question. Our research has shown that with the mixture of isobutene and normal butene, the oligomerization takes place at about 250°F. If pure propene is used, that temperature is at least 275°F. In case the esters leave the reactor, they are decomposed into phosphoric acid and you may see corrosion downstream.
SUGG (Honeywell UOP)
Our response is similar to Bryan’s, but there are a couple differences. We summarized it here on this slide. The concern for ester formation is limited to the C3-only feeds when you have reactor temperatures less than 275°F. As a little bit of history, this concern with low temperature operation with SPA catalyst was raised by UOP scientists back in the 1930s. Our early experiments show that the C3 oligomerization occurred readily at 275°F or higher. However, once the temperature was lowered to 257°F, the stable esters were formed which blocked the catalyst acid sites.
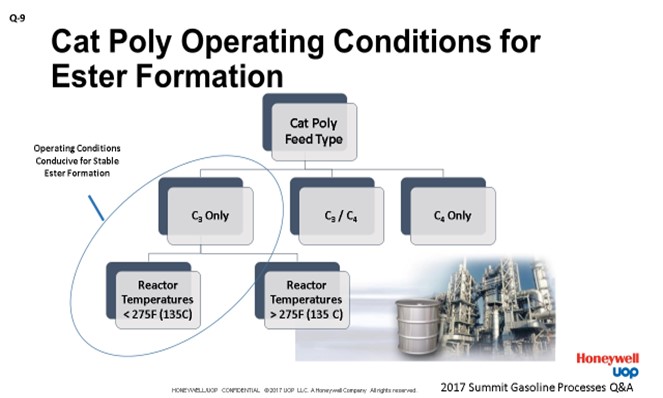
Because the presence of even a small amount of C4 olefins prevents the stable ester formation, it is only the C3 units that have to be concerned. UOP has a guideline to maintain C3 olefin catalytic polymerization operation above 300°F. We feel this is reasonable because it provides a margin of safety to ensure that no part of the catalyst bed operates below 275°F (considering the recycle and quench gas flows which impact the inlet and bed temperatures). If you get a low-temperature ester formation situation, you are going to get a subsequent loss in the conversion, which will require higher temperatures to maintain your desired product yields. As Bryan said, once the esters are formed, they are very corrosive; so, you have to be very careful handling them after that.
BRYAN KINDERMAN (Clariant Corporation)
The mechanism by which oligomerization takes place over the SPA catalyst necessitates that a phosphate ester is formed between the catalyst and the olefin. The temperature required to dissociate the phosphate ester and convert the olefin depends on the olefin in question. Research on phosphoric acid as a catalyst shows that a mixture of isobutene and n-butene can be oligomerized at 250°F. Pure propene was found to require at least 275°F.
In case phosphate esters leave the reactor, they are decomposed to phosphoric acid which causes corrosion in downstream equipment.
MARTHA BUCHAN (Honeywell UOP)
Ester formation in a catalytic polymerization (cat poly) unit is an issue for units with a C3 olefin-only feed, as the presence of even a small amount of C4 olefins prevents stable ester formation. With a C3 olefin feed, low reaction temperature is conducive to ester formation. The Honeywell UOP guideline is to avoid reactor operation below about 300°F (150°C). The concern with low-temperature operation causing the loss of stable phosphoric acid ester from the SPA catalyst was reported back in early UOP work (Ipatieff) in the 1930s. C3 olefin oligomerization was reported at 275°F (135°C) or higher reactor temperature; but at the slightly lower temperature of 257°F (125°C), the isopropyl ester will not decompose. The result was that C3 olefin oligomerization would not proceed at all; the stable esters will occupy all catalyst acid sites. This phenomenon was demonstrated in an autoclave reactor using propylene and 90% phosphoric acid. No polymer layer was formed at 257°F (125°C). The critical decomposition temperature for the phosphoric acid ester in the presence of propylene is somewhere between 257 and 275°F (125 and 135°C). If just a small amount of the stable phosphoric acid ester desorbs to the hydrocarbon, there could be a major corrosion problem.
Cat poly units with a C4 olefin only feed do not form the stable phosphoric acid esters at such high temperatures, as occurs with the C3 olefin feed; so, a C4 olefin feed will show reactivity over SPA, even at relatively low temperatures. Presence of just a small amount of C4 olefin makes apparent C3 olefin reactivity look higher for a C3/C4 feed, because presence of C4 olefin (competitive adsorption) prevents the stable C3 olefin ester from occupying all of the acid sites as it would in 100% C3 olefin feed.
The UOP Guideline to avoid C3 olefin cat poly operation below 300°F (150°C) is a reasonable general guideline to make sure that no part of the catalyst is below 275°F (135°C), considering there are recycle and quench flows impacting the inlet temperature and the exotherm in each bed. This guideline provides some safety margin from the anticipated condition where acid loss and resulting corrosion will occur.
Although UOP has no confirmed experience with ester formation in a C3-only cat poly unit, the expected impact on the process would be removal or blocking of active catalyst sites, reducing the effective catalyst activity. The reduction in conversion would require higher temperatures to maintain desired conversion levels. In addition, the stable phosphoric acid ester is highly corrosive, due to the presence of water, which will potentially create a major corrosion problem.