Question 16: HCl concentration in the reformer net off-gas is being actively measured using hand-held detector tubes. Despite routinely measuring near-zero ppm (parts per million), chloride corrosion occurs downstream. What is the source of this corrosion, and what can be done to better measure and manage the source?
SABITOV (Phillips 66)
One of the challenges with monitoring Cl trap performance is detecting organic chlorides. Organic chlorides may decompose downstream of the reformer and lead to corrosion problems. Therefore, we typically practice scheduled chloride treater changeouts. We believe that organic chlorides are formed because of the recombination of HCl and light olefins in reforming reactor effluent, although we acknowledge an ongoing debate as to whether organic Cl can be formed simply in reactor effluent system or would only form across spent Cl trap adsorbent.
Our sites are relying on expertise and analytical support of our adsorbent supplier for the detection of organic chlorides. In general, we are aware of two detection techniques: First is the thermal decomposition method where sample gas is heated to break down organic Cl to HCl so that total Cl concentration can be measured with a regular HCl detector tube. Then, organic Cl concentration is calculated as the delta between total Cl concentration and HCl concentration, as determined by conventional detector tube measurement of sample gas. The second method we are also evaluating is the use of light organic Cl detector tubes which come with catalyst in the pre-tube to breakdown organic Cl; and then similar to the first method, total Cl and organic Cl are determined.
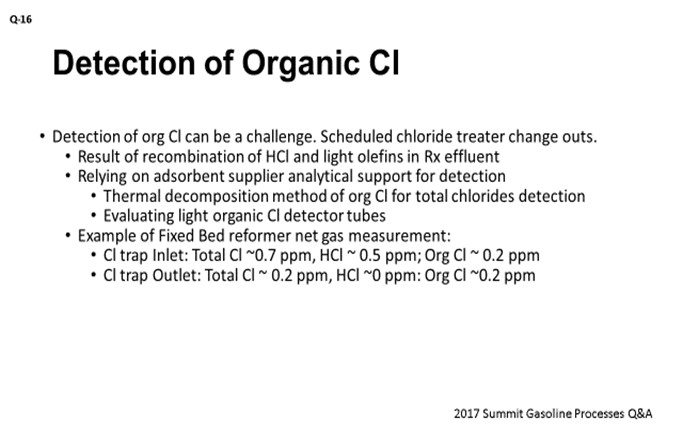
On the slide is a case study that shows some net gas chloride measurements using the first method in one of our fixed-bed reformers. Total Cl was measured at 0.7 ppm at the inlet of Cl treater and HCl was measured at 0.5 ppm, leaving a delta of 0.2 ppm for organic Cl. The same treater at the outlet had 0.2 ppm of total Cl and no HCl, leaving 0.2 ppm for organic Cl. This data might suggest that organic Cl might be breaking through the treater bed or potentially be formed across the treater bed.
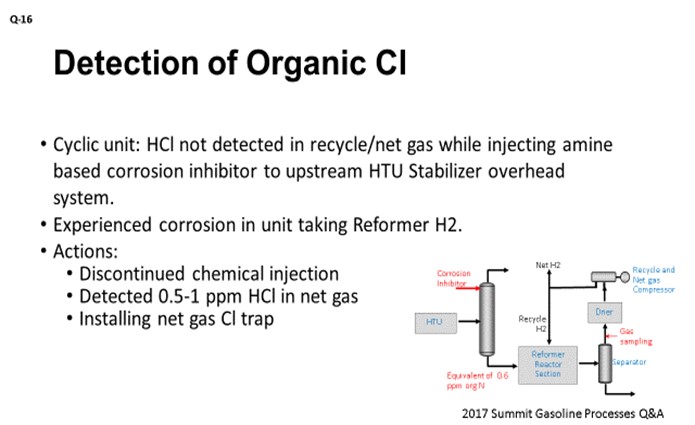
The other challenge with measuring Cl in the net gas can be due to interference with ammonia. We had an interesting case which can describe such situation. The cyclic reformer was injecting the amine-based corrosion inhibitor to the upstream HTU stabilizer overhead system at the rate which may bring up organic N (nitrogen) in the stabilizer bottoms (reformer feed) to about 0.6 ppmw. At the same time, we had difficulty detecting any HCl in various sections of the reformer recycle gas loop, even though it is a cyclic unit. From its original construction, the unit did not have a Cl trap. Despite there being no detected presence of HCl in recycle gas or net gas, the user of the reformer H2 experienced corrosion problems. We knew that reformer H2 should have at least trace of HCl, but we could not measure it. At that point, the site conducted a short test of discontinuing the corrosion inhibitor injection and immediately detected 0.5 to 1 ppmw HCl in the net gas that was using the detector tube. We are now proceeding with the installation of the chloride trap. It is still not clear whether NH3 (ammonia) is just interfering with the detector tube catalyst or if it, in fact, reacts with HCl in the process gas tying it as NH4Cl (ammonium chloride).
BRYAN KINDERMAN (Clariant Corporation)
This topic will also be covered in the Gasoline P&P session. Downstream issues have historically been tied to inorganic chlorides. Organic chlorides can be invisible to simple HCl- detecting tubes. There are alternative measuring devices from which to choose to detect organic chlorides. It is well known that organic chlorides can be created from an alumina guard bed and cause green oil, chloride salt formation, and corrosion downstream. There are non-alumina-based guard materials that significantly reduce the formation of organic chlorides and green oils.
RICHARD TODD (Norton Engineering Consultants, Inc.)
Chloride and moisture injection rates should be set on a per-weight of feed basis and should not be adjusted based on the net gas HCl concentration. It is possible that downstream corrosion is in an area where NH4Cl deposition occurs. Maintaining correct moisture injection levels and minimizing nitrogen content in the feed to less than detectable (i.e., less than 0. 2 ppm) will help to minimize downstream corrosion.
SAJEESH PADMANABHAN (Honeywell UOP)
The gas exiting the reactor section of a Platforming™ unit will always contain a certain amount of chloride. Most of that chloride will exist as HCl, though some of the HCl will combine with light olefins in the gas stream to form organic chlorides. Several operating factors may tend to increase the amount of HCl and organic chlorides in the net gas from the platforming unit. The amount of chloride stripped from the catalyst surface will increase as the moisture in the recycle gas increases. Also, as the catalyst ages and the surface area decline, the chloride retention decreases resulting in an increase in the chloride in the net gas. And the number of olefins in the reactor effluent will increase as the last reactor temperature increases at end-of-run (for semi-regen units) or high severity operations.
If the off-gas system does not contain a chloride treater to remove the chlorides, consider adding one. If a chloride treater is present, there may be premature breakthrough of chlorides through the bed; the adsorbent that is used may not be removing the organic chloride compounds; or, it may even be catalyzing their formation when it becomes saturated with chloride if it becomes acidic. Proper selection of the adsorbent in the chloride treater is important, as is timely replacement of the adsorbent, as it reaches its capacity.
The organic chlorides exist in equilibrium with HCl and olefins, so there will always be trace amounts of HCl in the downstream piping if organic chlorides are present. Wherever moisture is also present, and the temperature falls below the dew point, liquid water will form; the HCl will dissolve in that water, resulting in low pH and corrosion. Also, the organic chlorides will decompose into HCl and olefins at elevated temperatures in the downstream units.
It must also be noted that detection of organic chlorides in the net gas stream using gas detector tubes can be difficult. Some tubes are for HCl only; others react to some organic chlorides but not all; and some appear to work for a broader range of species. Honeywell UOP recommends that you discuss the issue with the manufacturer of the gas detector tubes that you use. Please note that UOP does not endorse a specific type of detector tube. Instead, we recommend that the user determine the changeout frequency of the chloride treater adsorbent based on the calculated chloride loading on the alumina.
You can also discuss the problem with your UOP service contacts for additional information about our experience.