Question 11: In chlorided isomerization units, have you seen evidence of unconverted perchloroethylene (PERC) in the stabilizer bottoms stream?
SABITOV (Phillips 66)
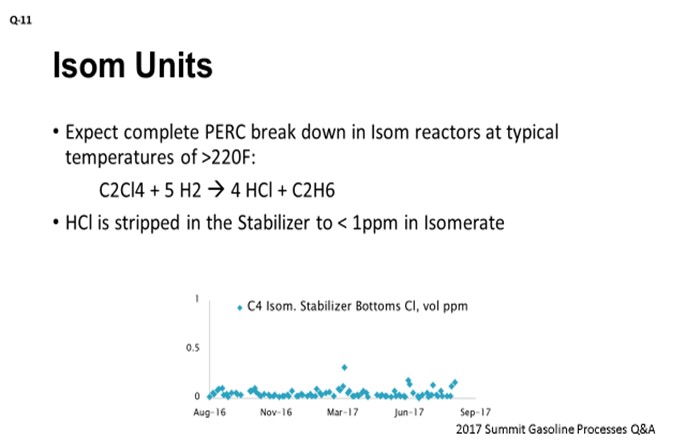
We expect full PERC breakdown on catalyst in the isomerization reactors. At typical isomerization reactor temperature above 220°F, PERC should be decomposing. This reaction on the slide shows that it is forming HCl and ethane. HCl should then be stripped in the isomerization stabilizer tower, with the result of very low chloride levels (less than 1 ppm) in the stabilizer bottoms. To demonstrate that scenario, I have included the graph showing total chloride in the stabilizer bottoms of our C4 isomerization unit. You can see that it is all well below 0.5 ppm and that the reactors in this unit are running roughly at 280°F.
Having said that, just to make it interesting, we recently encountered a case where one of our refinery labs started reporting about 2 to maybe 2.6 ppm total chloride in the stabilizer bottoms of the light naphtha isomerization unit. We performed an analytical investigation and determined that for the same samples, the inorganic chloride was about 0.2 to 0.6 ppm, which we assumed was HCl. We did some field tests of the isomerate vapor phase using HCl detector tubes and did not detect HCl, which was consistent with trace level for HCl reported by the lab. We are thinking that the 2-ppm difference between total chloride and inorganic chloride falls on PERC, which we believe is bypassing the reactors through one of the CFEs, which is potentially leaking. This unit is still in operation pending turnaround. Once we have those exchangers opened and inspected, we will see whether we can prove or disprove our theory.

DUBIN (Axens North America)
Like Alex, we have some operating experience with what is inferred to be organic chlorides in the product, with a similar result of the total chlorides being quite a bit higher than the inorganic chlorides, which directionally indicates that there are some organic chlorides hanging around in the product. Investigating the system circuit, it was observed that the temperatures were fully sufficient to decompose PERC, so a leak or some other reaction mechanism is being proposed for the situation.
GEOFFREY DUBIN (Axens North America, Inc.)
Axens has evidence of the presence of organic chlorides in the isomerization stabilizer bottoms product. Since testing specifically for organic chlorides is not possible, one must compare the total chloride content of the isomerate against the HCl content to determine derived organic chloride content. It can be inferred that the difference in the two numbers – total chlorides and HCl – is organic chlorides and that the organic chloride is undecomposed PERC. The operating temperature of the isomerization reactors should be sufficient to decompose PERC. The apparent presence of organic chlorides in the isomerate indicates that the reaction mechanism is not complete.
JOCELYN DAGUIO (Honeywell UOP)
There have been reported incidents of unconverted perchloroethylene (PERC) in the stabilizer bottoms of Honeywell UOP Penex™ and Butamer™ process units. The most likely cause is compromised metal (platinum) sites on the catalyst due to the presence of contaminants that entered the unit. Sulfur, CO, or CO2 from either the feed or makeup gas are the most likely of these contaminants.
PERC decomposes to HCl and ethane in the presence of H2 over Penex or Butamer catalyst at reactor temperature of at least 220°F (105°C). However, only the metal sites on the Penex or Butamer catalyst is needed for the reaction to proceed. If the metal sites are attenuated by metal poisons, the reaction will not proceed. Any unconverted PERC will drop down the stabilizer column with the isomerate.
Once the poisons have been removed from the metal sites, the reaction can proceed. With all PERC completely decomposed, HCl will leave with the stabilizer off-gas for neutralization in the scrubber.